What to Do With N in Degrees of Unsaturation Princeton Review
viii.4: Degrees of Unsaturation
- Folio ID
- 45189
Learning Objectives
- summate the Degrees of Unsaturation (DU) and utilise information technology to alkene structure
Saturated and Unsaturated Molecules
In the lab, saturation may be thought of equally the bespeak when a solution cannot dissolve anymore of a substance added to it. In terms of degrees of unsaturation, a molecule but containing single bonds with no rings is considered saturated.
Different saturated molecules, unsaturated molecules contain double bond(s), triple bond(southward) and/or band(south).
| CH3CH=CHCHthree | 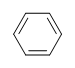 | 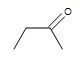 | 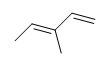 | 3-chloro-5-octyne |
In that location are many ways one tin can go most determining the structure of an unknown organic molecule. Although, nuclear magnetic resonance (NMR) and infrared radiations (IR) are the primary means of determining molecular structures, these techniques require expensive instrumentation and are not ever readily bachelor. Fortunately, calculating the degrees of unsaturation provides useful data most the construction. The degree of unsaturation indicates the total number of pi bonds and rings within a molecule which makes it easier for one to figure out the molecular structure.
DU = Degrees of Unsaturation = (number of pi bonds) + (number of rings)
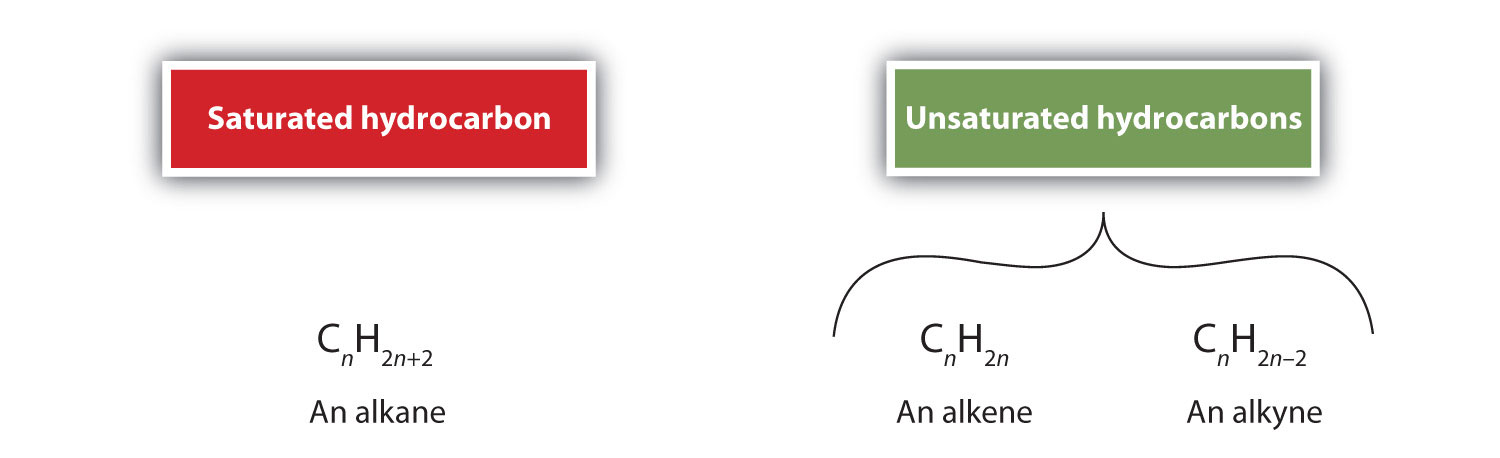
Alkenes (R2C=CRtwo) and alkynes (R–C≡C–R) are called unsaturated hydrocarbons because they have fewer hydrogen atoms than does an alkane with the same number of carbon atoms, as is indicated in the post-obit general formulas:
Calculating The Degree of Unsaturation (DU)
If the molecular formula is given, plug in the numbers into this formula:
\[ DoU= \dfrac{2C+two+N-Ten-H}{2} \tag{7.ii.1}\]
- \(C\) is the number of carbons
- \(North\) is the number of nitrogens
- \(X\) is the number of halogens (F, Cl, Br, I)
- \(H\) is the number of hydrogens
The molecular formula of a hydrocarbon provides information about the possible structural types it may represent. A saturated molecule contains only single bonds and no rings. Another way of interpreting this is that a saturated molecule has the maximum number of hydrogen atoms possible to exist an acyclic paraffin. Thus, the number of hydrogens tin can be represented past 2C+two, which is the full general molecular representation of an alkane series. As an example, for the molecular formula C3H4 the number of actual hydrogens needed for the compound to be saturated is viii
[2C+two=(2x3)+2=8.]
The chemical compound needs 4 more hydrogens in guild to be fully saturated (expected number of hydrogens-observed number of hydrogens=8-4=iv). Degrees of unsaturation is equal to 2, or half the number of hydrogens the molecule needs to be classified as saturated. Hence, the DoB formula divides past ii. The formula subtracts the number of X'southward because a halogen (X) replaces a hydrogen in a compound. For instance, in chloroethane, C2H5Cl, there is 1 less hydrogen compared to ethane, C2H6. For example, consider compounds having the formula C5H8. The formula of the 5-carbon alkane pentane is C5H12 and then the departure in hydrogen content is 4. This deviation suggests such compounds may take a triple bail, two double bonds, a ring plus a double bond, or two rings. Some examples are shown here, and there are at least fourteen others!
For a compound to be saturated, there is ane more hydrogen in a molecule when nitrogen is nowadays. Therefore, we add the number of nitrogens (N). This can be seen with CthreeHixN compared to C3H8. Oxygen and sulfur are not included in the formula because saturation is unaffected by these elements. Equally seen in alcohols, the same number of hydrogens in ethanol, CiiHfiveOH, matches the number of hydrogens in ethane, C2H6.
The following chart illustrates the possible combinations of the number of double bond(s), triple bond(s), and/or ring(due south) for a given caste of unsaturation. Each row corresponds to a different combination.
- One caste of unsaturation is equivalent to 1 ring or 1 double bond (ane \( \pi \) bond).
- Two degrees of unsaturation is equivalent to 2 double bonds, one band and 1 double bond, 2 rings, or 1 triple bond (2 \( \pi \) bonds).
When the DU is 4 or greater, the presence of benzene rings is very likely.
| DU | Possible combinations of rings/ bonds | ||
|---|---|---|---|
| # of rings | # of double bonds | # of triple bonds | |
| 1 | 1 | 0 | 0 |
| 0 | 1 | 0 | |
| 2 | ii | 0 | 0 |
| 0 | 2 | 0 | |
| 0 | 0 | 1 | |
| ane | 1 | 0 | |
Retrieve, the degrees of unsaturation only gives the sum of double bonds, triple bonds and/or rings. For instance, a caste of unsaturation of iii can comprise 3 rings, 2 rings+ane double bail, 1 ring+2 double bonds, 1 band+one triple bail, one double bail+1 triple bail, or 3 double bonds.
Example: Benzene
What is the Caste of Unsaturation for Benzene?
Solution
The molecular formula for benzene is CviH six . Thus,
DU= iv, where C=6, Due north=0,X=0, and H=6. 1 DoB can equal one ring or i double bail. This corresponds to benzene containing 1 band and iii double bonds.

However, when given the molecular formula C6Hhalf-dozen, benzene is only 1 of many possible structures (isomers). The post-obit structures all have DU of 4 and accept the aforementioned molecular formula equally benzene. Withal, these compounds are very rare, unlike benzene. We will learn more than about the reasons for benzen'southward high stability when we studey aromaticity in afterwards capacity.

Exercises
- Are the following molecules saturated or unsaturated:
-
 (b.)
(b.) (c.)
(c.) 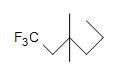 (d.) CxH6Due north4
(d.) CxH6Due north4
-
- Using the molecules from (one) above, give the degrees of unsaturation for each.
- Calculate the degrees of unsaturation, classify the chemical compound as saturated or unsaturated, and list all the ring/pi bond combination possible for the following molecular formulas: (a.) CnineH 20 (b.) C7H 8 (c.) C5H7Cl (d.) CnineH9NOiv
- Calculate degrees of unsaturation (DoU) for the following, and propose a construction for each.
a) C5Height
b) CfourH4
-
Calculate the degree of unsaturation (DoU) for the following molecules
a) C5HvN
b) C5H5NO2
c) C5H5Br
-
The following molecule is caffeine (C8H10N4Oii), determine the degrees of unsaturation (DoU).
.png?revision=1)
- Answer
-
1.
(a.) un saturated (Even though rings only incorporate single bonds, rings are considered unsaturated.)
(b.) unsaturated
(c.) saturated
(d.) unsaturated
two. If the molecular structure is given, the easiest fashion to solve is to count the number of double bonds, triple bonds and/or rings. All the same, yous tin also determine the molecular formula and solve for the degrees of unsaturation past using the formula.
(a.) two
(b.) 2 (one double bail and the double bond from the carbonyl)
(c.) 0
(d.) 10
three.
(a.) DU =0 ; saturated (Remember-a saturated molecule only contains single bonds)
(b.) DU = iv; unsaturated The molecule tin contain whatever of these combinations of rings and pi bonds that add upwardly to four, such as (i) 4 double bonds (ii) 4 rings (iii) 2 double bonds+2 rings (iv) 1 double bond+3 rings (v) three double bonds+one ring (vi) 1 triple bail+2 rings (vii) 2 triple bonds (viii) 1 triple bond+one double bond+1 ring (ix) 1 triple bond+2 double bonds
(c.) DU = 2; unsaturated (i) 1 triple bail (two) 1 ring+1 double bond (three) ii rings (iv) two double bonds
(d.) DU = vi; (i) 3 triple bonds (ii) ii triple bonds+2 double bonds (iii) 2 triple bonds+i double bond+1 ring (iv)... (As you can see, the degrees of unsaturation merely gives the sum of double bonds, triple bonds and/or band. Thus, the formula may give numerous possible structures for a given molecular formula.)
4.

5. a) 4 b) four c) 3
half dozen. DU = 6
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(Wade)/08%3A_Structure_and_Synthesis_of_Alkenes/8.04%3A_Degrees_of_Unsaturation
0 Response to "What to Do With N in Degrees of Unsaturation Princeton Review"
Post a Comment